What is HIB? “Haemophilus influenzae disease is a name for any illness caused by bacteria called H. influenzae. Some of these illnesses, like ear infections, are mild while others, like bloodstream infections, are very serious. In spite of the name, H. influenzae do not cause influenza (the flu).” (cdc.gov) These bacteria live in people’s nose and throat, and usually cause no harm. However, the bacteria can sometimes move to other parts of the body and cause infection. (cdc.gov)
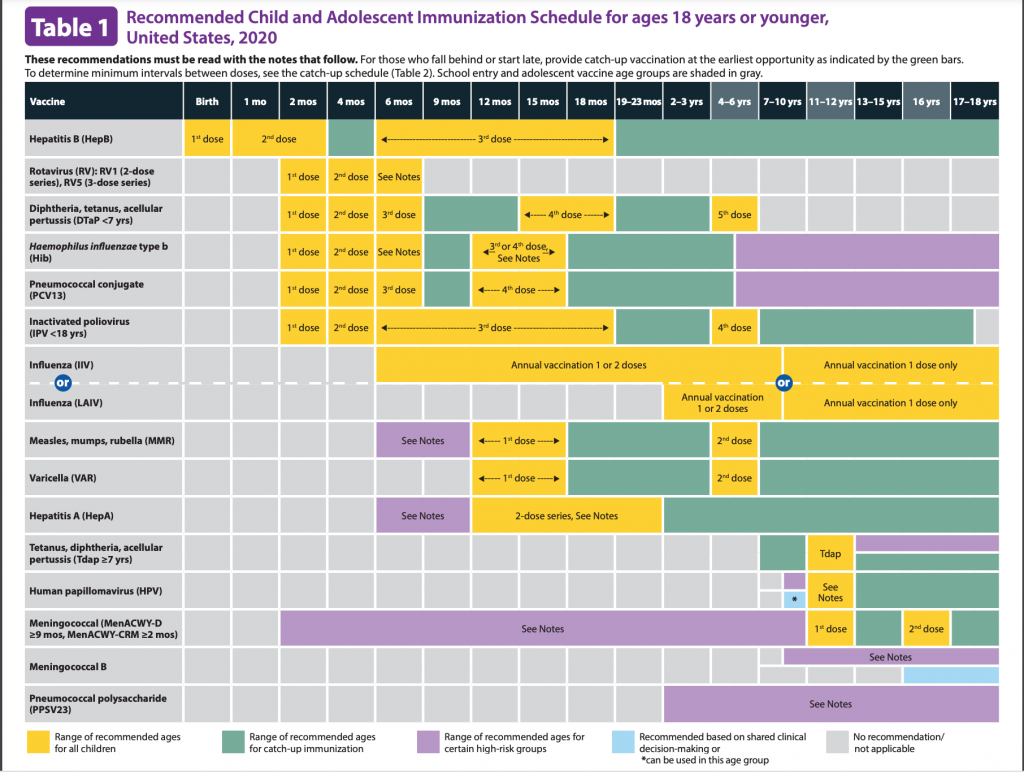
When is HIB vaccination recommended? Here is the cdc vaccine schedule. It is usually a 4 dose series: 2mo, 4mo, 6mo, 12-15months for ActHIB, Hiberix, or Pentacel. It is a 3 dose series for PedvaxHIB.
So how many cases per year are there? “Today, less than 50 cases of Hib disease occur each year in young children in the United States. Most of these cases are in children who did not get any or all recommended doses of Hib vaccine.” (cdc.gov/vaccines)
According to the cdc if there are less than 50 cases a year of Hib, divide the population 331 million in the US by 50 and your chances of getting Hib today in the US would be 1 in 6,620,000. Wow that is pretty low. What do you think your chances are of having an adverse reaction to the vaccine?
How many adverse reactions have been reported to VAERS from HIB? I was curious so I took a look. I found 48,067 adverse reactions reported from the HIB vaccine (from 1990 to present day.)
Have you ever checked out VAERS? I will cover how to do this more in a future post. But to put it simply you can go to the VAERS website and after clicking agree to disclaimer you can hit VAERS data search button in the middle, to search all the adverse events by vaccine or by reaction.
Now let’s look at one of the vaccine inserts for Hib…
Can there be adverse reactions to the vaccine? “The use of Haemophilus b Polysaccharide Vaccines and another Haemophilus b Conjugate Vaccine has been associated with the following additional adverse effects: early onset Hib disease and Guillain-Barré syndrome.” Febrile seizures were also a side effect post marketing. (Liquid PedvaxHIBinsert) So you could get the disease, get seizures, or worse become paralyzed from the vaccine.
Here are some precautions, ” As for any vaccine, adequate treatment provisions, including epinephrine, should be available for immediate use should an anaphylactoid reaction occur….cases of Hib disease may occur in the week after vaccination.” Our common theme here too, “Liquid PedvaxHIB has not been evaluated for carcinogenic or mutagenic potential, or potential to impair fertility.” (Liquid PedvaxHIBinsert)
How protective is this vaccine against HIB infections? “Liquid PedvaxHIB will not protect against disease caused by Haemophilus influenzae other than type b or against other microorganisms that cause invasive disease such as meningitis or sepsis. As with any vaccine, vaccination with Liquid PedvaxHIB may not result in a protective antibody response in all individuals given the vaccine. (Liquid PedvaxHIBinsert)
From a study in the insert, how long do you think they monitored for adverse effects from HIB vaccination? Did they monitor the children for years? Months? Weeks? NO 3 DAYS!!! “During a three-day period following primary vaccination with Liquid PedvaxHIB in these infants, the most frequently reported (>1%) adverse reactions, without regard to causality, excluding those shown in TABLE 5, in decreasing order of frequency, were: irritability, sleepiness, injection site pain/soreness, injection site erythema (2.5 cm diameter, see also TABLE 5), injection site swelling/induration (2.5 cm diameter, see also TABLE 5), unusual highpitched crying, prolonged crying (>4 hr), diarrhea, vomiting, crying, pain, otitis media, rash, and upper respiratory infection. 18.1% also had a fever after the first dose and 14.1% had one after the second dose. (Liquid PedvaxHIBinsert). Refer to the table below for rates of fevers, erythema, and swelling.
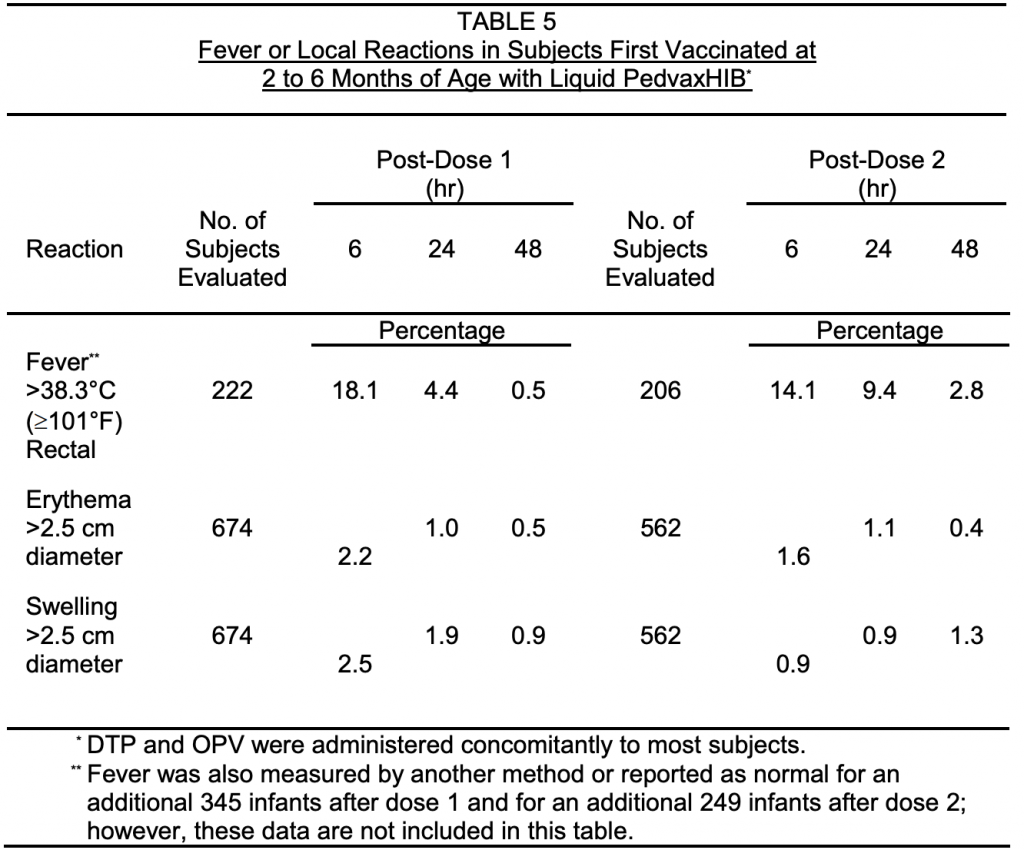
So it’s good to look at the risk of getting the disease, which we just covered in the US it is very low. It is important to compare the risk of the disease to the risk for adverse reactions from the vaccine to make an informed decision! Seems like your chances of adverse reaction are pretty high and risk for this disease is low. Now you can decide if your child needs 3 or 4 doses of this vaccine….